Unwanted Fc-mediated effector functions
Antibodies have evolved to target pathogens and activate the host’s immune system. Engagement of the immune system in this way through interaction with Fc receptors and complement factor C1q results in targeted cell death via a number of different mechanisms: ADCC, ADCP and CDC. For oncology indications this antibody mediated cell death is highly desirable, in fact a number of different technologies have been developed to enhance so-called Fc effector function. Alternatively, therapeutic antibodies can simply act as blocking agents to prevent the interaction of a ligand and its’ receptor. In this context Fc effector function is unnecessary, and worse it can be highly immunogenic or toxic. In mild cases this can result in enhanced clearance and neutralisation of the drug by anti-drug antibodies, thus reducing its efficacy. In more serious cases engagement with Fc receptors can activate the immune response leading to cytokine release syndrome (CRS), organ failure and possible death.
For this reason, it is now commonplace for therapeutic antibodies that do not utilise antibody mediated cell killing as a primary mode of action to be engineered to have ablated effector function – often known as silencing. In fact, it is our observation that approximately 50% of therapeutic antibodies currently in clinical development utilise some form of silencing. There are a number of well characterised approaches for silencing, but all of them suffer the same flaw - they aren’t truly silent. It was this simple observation that all available technologies retain residual binding to Fc receptors, and by inference a degree of toxicity risk, that led us to develop a novel and truly silent Fc domain.
STR is a novel combination of three mutations in the CH2 domain of the Fc. This was developed from a screen of over 300 variant Fcs with two specific goals in mind. First, to obtain an Fc domain with minimal binding to Fc receptors and C1q, ideally totally silent within our detection limits. Second, to obtain an Fc domain that in all other respects maintained the functionality and developability of a wild type Fc domain, for instance stability, Protein A binding and half-life. With STR we have achieved this goal, a truly silent and highly developable Fc.
To select the STR variant we went through an extensive screening process incorporating Fc receptor binding (SPR), C1q binding (ELISA), cell based FcγR reporter assays, FcRn binding (SPR), in vivo PK studies, in silico and in vitro immunogenicity analysis, thermal stability and forced degradation amongst many others. We are proud to report that some of this data has recently been published in the peer reviewed journal PLoS ONE and can be found here.
STR performed favourably against other Fc silencing technologies
We were keen to benchmark our STR variant against other reported Fc silencing technologies. In particular, L234A/L235A (commonly known as LALA) and aglycosylation are the two most widely used approaches, in part due to both being free of intellectual property. As has been reported by many others (insert links to papers), both of these variants retain considerable levels of residual binding to Fc receptors. In contrast, we have been unable to detect significant levels of binding above background noise for STR in any assay we have yet employed, as exemplified in figure 1.
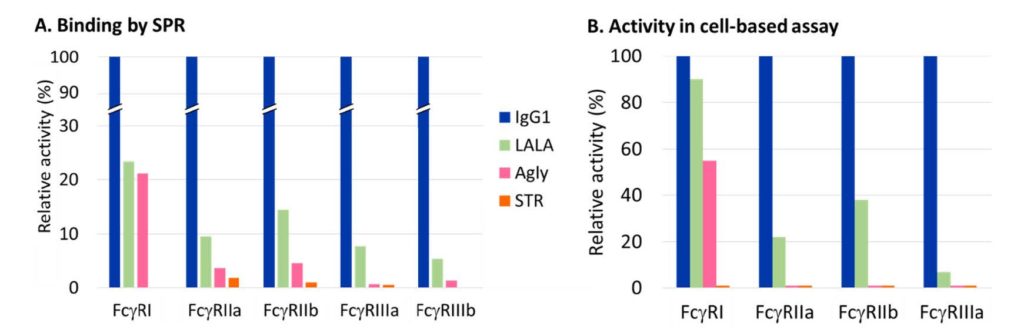
Figure 1. Analysis of binding to Fc receptors in vitro by surface plasmon resonance (SPR, Biacore) [A] and in cell based reporter assays [B]. STR is compared with wild-type IgG1 and the most commonly used silencing variants LALA and aglycosylated. In all cases the STR variant shows no significant binding above the background noise of the assay, whereas LALA and Agly show considerable levels of residual binding to some classes of Fc receptor.
Further comparisons showed STR to perform better with no detectable binding
We have then taken this a step further and compared STR to all reported silencing technologies known to us at the time of the assay (figure 2). We measured binding to the high affinity receptor FcγRI (CD64) to obtain the greatest sensitivity. Not only is STR a major improvement over the widely utilised IP-free options, it is the only variant to demonstrate no detectable binding at all. This is despite many of the others being reported as “silent”, “no detectable binding” and “completely abolished”.
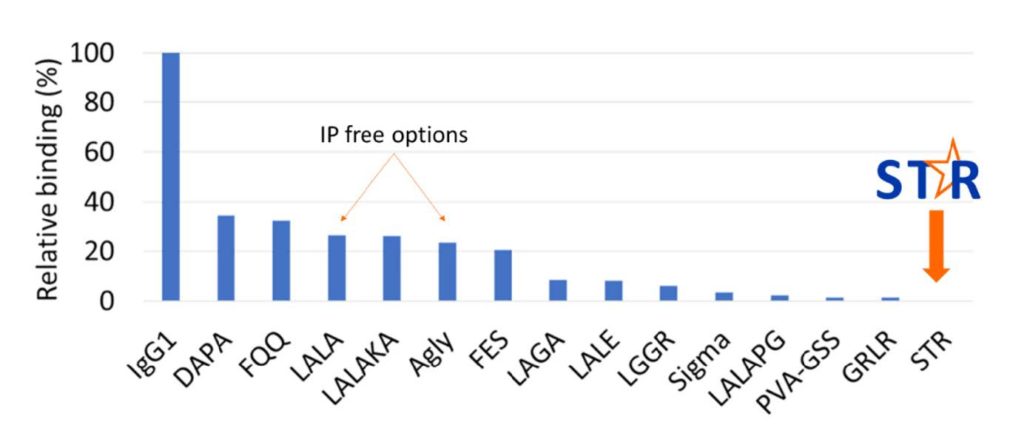
Figure 2. Comparison of silencing approaches in their relative binding to human FcRI (CD64) by surface plasmon resonance (SPR, Biacore). STR is the only variant to show no detectable binding.
Safer for patients
Our aim was to generate a safer option for Fc silencing, minimising the risk of cytokine release in patients. To determine if STR met this criteria we ran a cytokine release assay with human peripheral blood mononuclear cells (PBMCs) isolated from healthy donors (figure 3). Encouragingly, STR showed no significant activity above buffer alone for all cytokines measured. In contrast, LALA and aglycosylated antibodies both showed considerable levels of cytokine release, confirming our strong belief that these widely used silencing approaches are not optimal for safe and effective clinical development of antibodies or Fc fusion proteins were silencing is preferred.
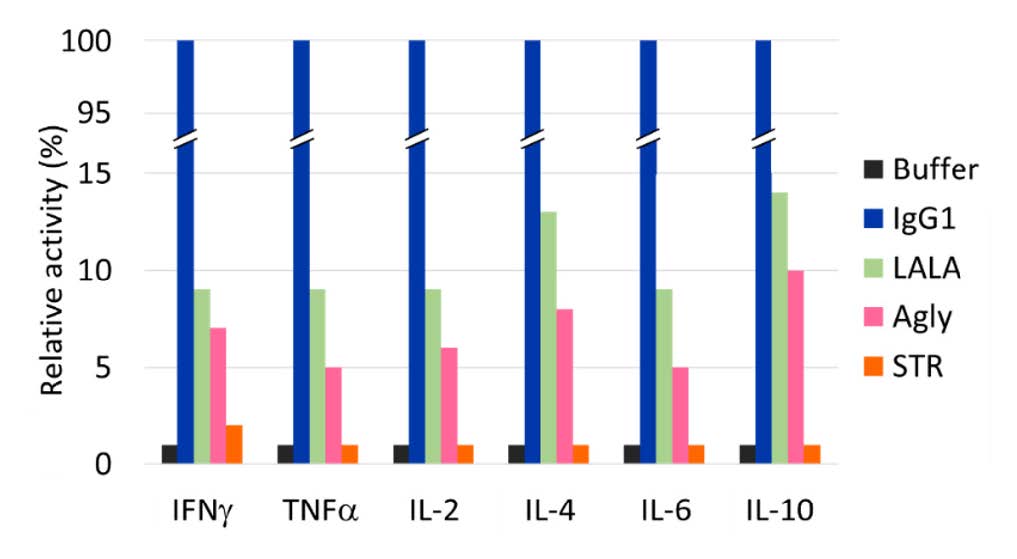
Figure 3. Release of cytokines from human PMBCs exposed to CD3 binding antibodies.
Licensing - STR technology is available to license
We are not involved in the research and development of our own biological therapeutics. Instead, it is our strong belief that the STR technology can be of real benefit to a large proportion of antibody and Fc fusion proteins currently being developed. We are therefore actively seeking to license our technology widely on affordable terms with non-exclusive research and commercial licenses available. Payments are based on a few simple success-based milestones with no royalties.
